Equilibrium - Le Chatelier's Principle Lab
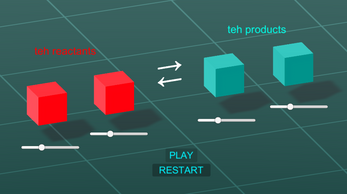
This is a quick chemistry simulation showing Le Chatelier's Principle: Use the sliders to change how much of each substance you want. You can increase or decrease the amount of reactants or products (slide the slider all the way to the left or right or else the simulation won't work. Also, choose only one item to change; the simulation won't do multiple items changing at once ). Then press play. This shows how the reactants/products adjust to the change (this is a "change in concentration"). The arrow in the middle is pointing in the direction that the equation shifts too.
Le Chatelier's Principle (per Wikipedia):
"Effect of change in concentration"
"Changing the concentration of a chemical will shift the equilibrium to the side that would counter that change in concentration. The chemical system will attempt to partly oppose the change affected to the original state of equilibrium. In turn, the rate of reaction, extent, and yield of products will be altered corresponding to the impact on the system.
"This can be illustrated by the equilibrium of carbon monoxide and hydrogen gas, reacting to form methanol.
CO + 2 H2 ⇌ CH3OH
"Suppose we were to increase the concentration of CO in the system. Using Le Chatelier's principle, we can predict that the concentration of methanol will increase, decreasing the total change in CO. If we are to add a species to the overall reaction, the reaction will favor the side opposing the addition of the species. Likewise, the subtraction of a species would cause the reaction to "fill the gap" and favor the side where the species was reduced. This observation is supported by the collision theory. As the concentration of CO is increased, the frequency of successful collisions of that reactant would increase also, allowing for an increase in forward reaction, and generation of the product. Even if the desired product is not thermodynamically favored, the end-product can be obtained if it is continuously removed from the solution."
[Source: https://en.wikipedia.org/wiki/Le_Chatelier%27s_principle ]
| Status | Released |
| Platforms | HTML5 |
| Author | Modulus Labs |
| Made with | Unity |
Development log
- chemistry is a goMay 09, 2021
Leave a comment
Log in with itch.io to leave a comment.